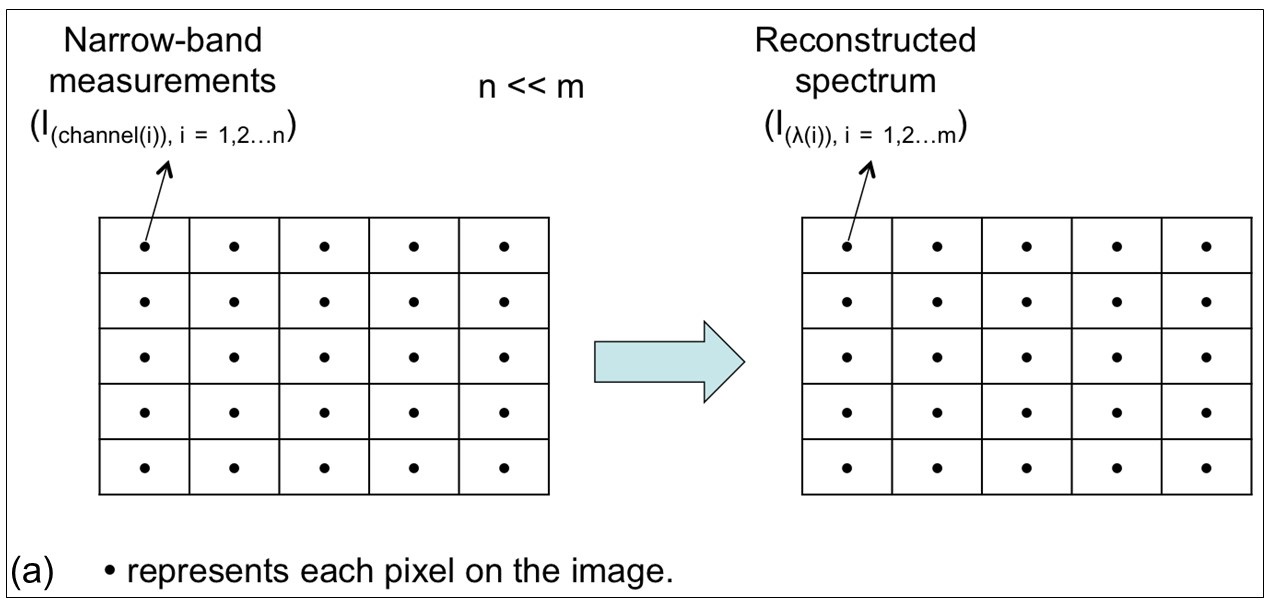
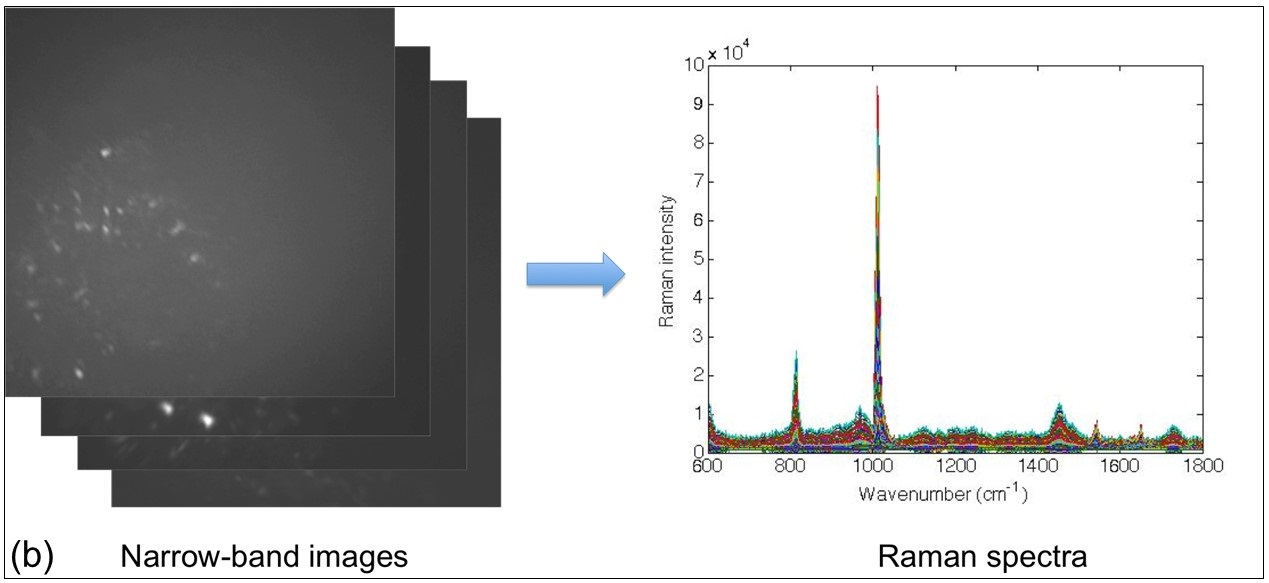
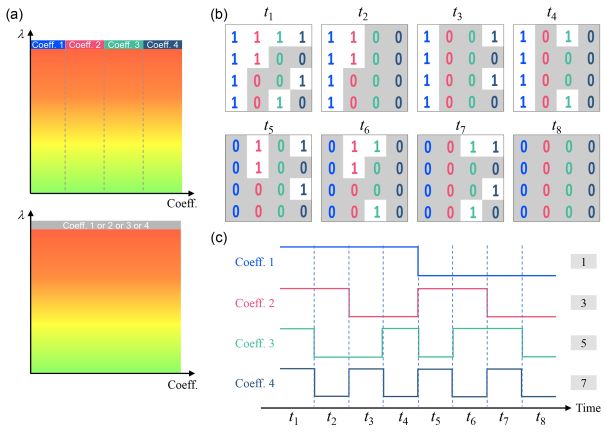
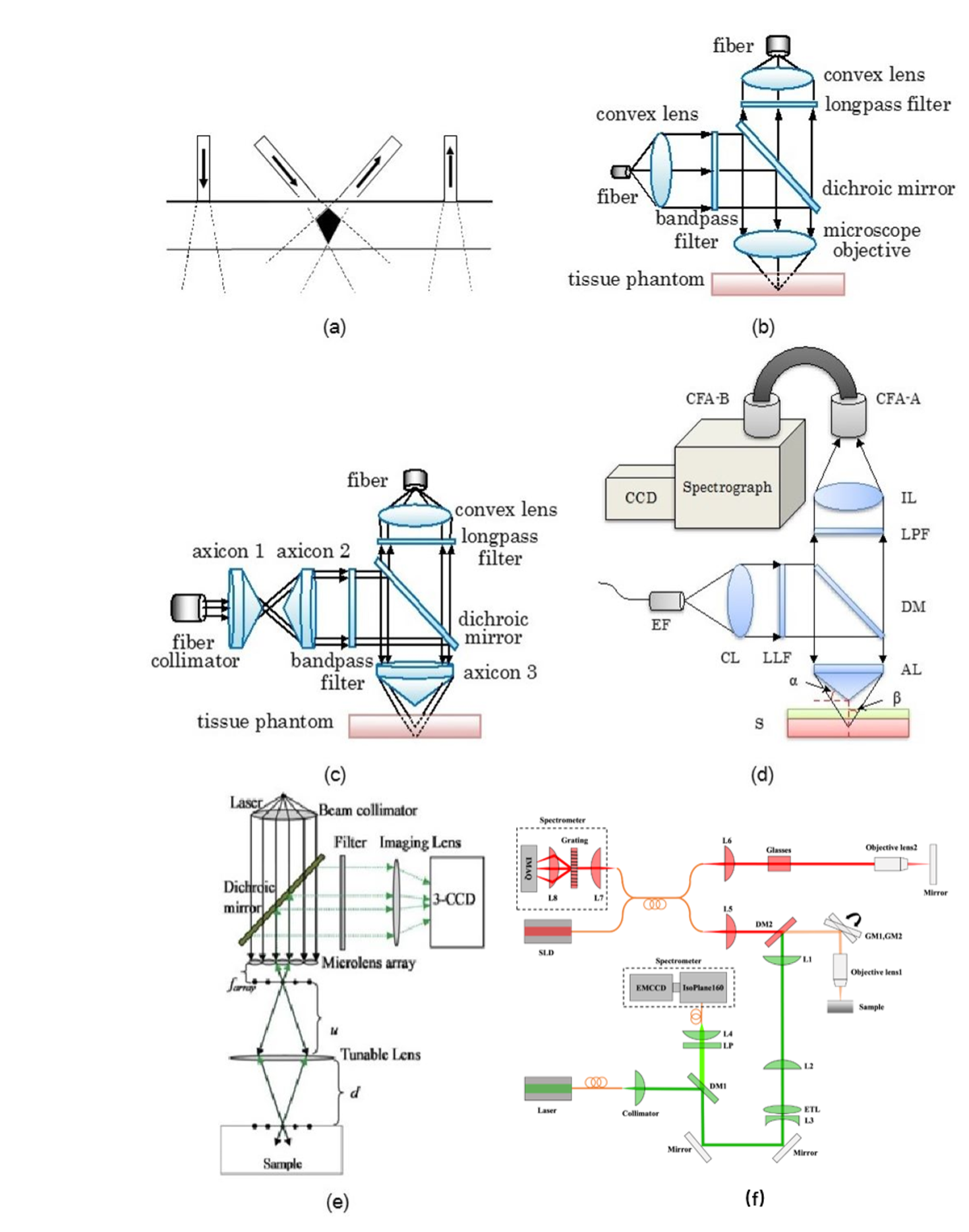
To overcome the limitation of confocal Raman microspectroscopy in measurement speed, we recently developed a hybrid system (Biomedical Optics Express 2022) as shown in Fig. 3(f), in which another much faster imaging technique, i.e. optical coherence tomography (OCT), is used to guide confocal Raman microspectroscopy for rapid measurements in tissues. An electrically tunable lens is used to quickly vary the focal depth of the Raman module to focus on the target identified from OCT images.
Relevant publications:
18. Xiaojing Ren, Kan Lin, Chao-Mao Hsieh, Linbo Liu, Xin Ge,* and Quan Liu*, "Optical coherence tomography guided confocal Raman spectroscopy for rapid measurements in tissues," Biomedical Optics Express, 13(1), 344-357 (2022).
17. Joshua Weiming Su, Qiang Wang, Yao Tian, Leigh Madden, David Laurence Becker, and Quan Liu*, "Depth-sensitive Raman spectroscopy for skin wound evaluation in rodents," Biomedical Optics Express, 10(12), 6114-6128 (2019).
16. Chao-Mao Hsie, Manish Verma, and Quan Liu*, "Improving depth sensitive fluorescence spectroscopy with wavefront shaping by spectral and spatial filtering," IEEE Access, 7, 170192-170198 (2019).
15. Wei Liu, Yi Hong Ong, Xiaojun Yu, Jian Ju, Clint Michael Perlaki, Linbo Liu and Quan Liu*, "Snapshot depth-sensitive Raman spectroscopy in layered tissues," Optics Express, 24(25), 28312-28325 (2016).
14. Caigang Zhu, Shuo Chen, Christopher Hoe-Kong Chui, Bien-Keem Tan and Quan Liu*, "Early detection and differentiation of venous and arterial occlusion in skin flaps using visible diffuse reflectance spectroscopy and autofluorescence spectroscopy," Biomedical Optics Express, 7(2), 570-580 (2016).
13. Fei Gao, Yi Hong Ong, Gaoming Li, Xiaohua Feng, Quan Liu, and Yuanjin Zheng, "Fast photoacoustic-guided depth-resolved Raman spectroscopy: a feasibility study," Optics Letters, 40(15), 3568-3571. (2015).
12. Yi Hong Ong, Caigang Zhu, and Quan Liu*, "Phantom Validation of Monte Carlo Modeling for Non-contact Depth Sensitive Fluorescence Measurements in an Epithelial Tissue Model," Journal of Biomedical Optics, 19(8), 85006 (2014).
11. C. Zhu, Y. H. Ong and Quan Liu*, "Multifocal noncontact color imaging for depth sensitive fluorescence measurements of epithelial cancer," Optics Letters, 39(11), 3250-3253 (2014).
10. C. Zhu, Shuo Chen, Christopher Hoe-Kong Chui, Bien-Keem Tan and Quan Liu*, "Early Prediction of Skin Viability using Visible Diffuse Reflectance Spectroscopy and Autofluorescence Spectroscopy," Plastic and Reconstructive Surgery, 134(2):240e-7e (2014).
9. Y. H. Ong, and Quan Liu*, "Fast depth-sensitive fluorescence measurements in turbid media using cone shell configuration," Journal of Biomedical Optics 18(11), 110503 (2013).
8. Yi Hong Ong and Quan Liu*, "Axicon lens-based cone shell configuration for depth-sensitive fluorescence measurements in turbid media," Optics Letters, 38(15), 2647-2649 (2013).
7. C. Zhu and Quan Liu*, "Review of Monte Carlo modeling of light transport in tissues," Journal of Biomedical Optics, 18(5), 050902 (2013).
6. C. Zhu and Quan Liu*, "Numerical investigation of lens based setup for depth sensitive diffuse reflectance measurements in an epithelial cancer model," Optics Express 20(28), 29807-29822 (2012).
5. C. Zhu and Quan Liu*, "A hybrid method for fast Monte Carlo simulation of diffuse reflectance from a multi-layered tissue model with tumor-like heterogeneities," Journal of Biomedical Optics 17(1), 010501 (2012).
4. C. Zhu and Quan Liu*, "Validity of the semi-infinite tumor model in diffuse reflectance spectroscopy for epithelial cancer diagnosis: a Monte Carlo study," Optics Express 19(18), 17799-17812 (2011).
3. Quan Liu and N. Ramanujam*, "Scaling method for fast Monte Carlo simulation of diffuse reflectance spectra from multi-layered turbid media," Journal of the Optical Society of America A, 24, 1011-1025 (2007).
2. Quan Liu, and N. Ramanujam*, "Sequential estimation of optical properties of a two-layered epithelial tissue model from depth-resolved ultraviolet-visible diffuse reflectance spectra," Applied Optics, 45, 4776-4790 (2006).
1. Quan Liu, and N. Ramanujam*, "Experimental proof of the feasibility of using an angled fiber-optic probe for depth-sensitive fluorescence spectroscopy of turbid media," Optics Letters 29, 2034-2036 (2004).
3. Surface enhanced Raman spectroscopy (SERS) for malaria diagnosis and minimally invasive microneedles for intradermal measurements
Malaria continues to be one major killer of humans, especially in developing regions. There is lack of effective procedures for early malaria diagnosis in the field. The creative approach developed in our group was the integration of magnetic enrichment with surface enhanced Raman spectroscopy (SERS), which improved the sensitivity of SERS by two orders of magnitude to near that of the standard clinical method (Journal of Biomedical Optics 2012, Analyst 2013 & US Patent Application No. 13/442,594). Our more recent ultrasensitive SERS technique, in which SERS active nanoparticles are synthesized inside parasites to increase the probability of forming hot spots, boosted up the sensitivity of SERS further to outperform the standard method (Scientific Reports 2016), which could lead to a new rapid diagnostic tool suitable for the field use. The sensitivities of other techniques (in green) and SERS techniques including ours (highlighted in yellow) are compared in Fig. 4. We have also shown that the new technique can be used to detect SERS spectra of hemozoin from individual parasites in the ring stage (IEEE JSTQE 2016) as shown in Fig. 5. To make this new technique suitable for deployment in a low-resource setting, we designed a transparency based fluidic chip (Sensors & Actuators: B 2021) as shown in Fig. 6 for on-chip sample preparation and near-analyte nanoparticle synthesis such that a central lab is no longer required for blood sample preparation. This is an important step towards malaria field diagnosis based on surface enhanced Raman scattering. We are currently developing a cost effective Raman spectrometer in an attempt to serve as a low-cost chip reader to prepare for this SERS technique to be adopted widely.
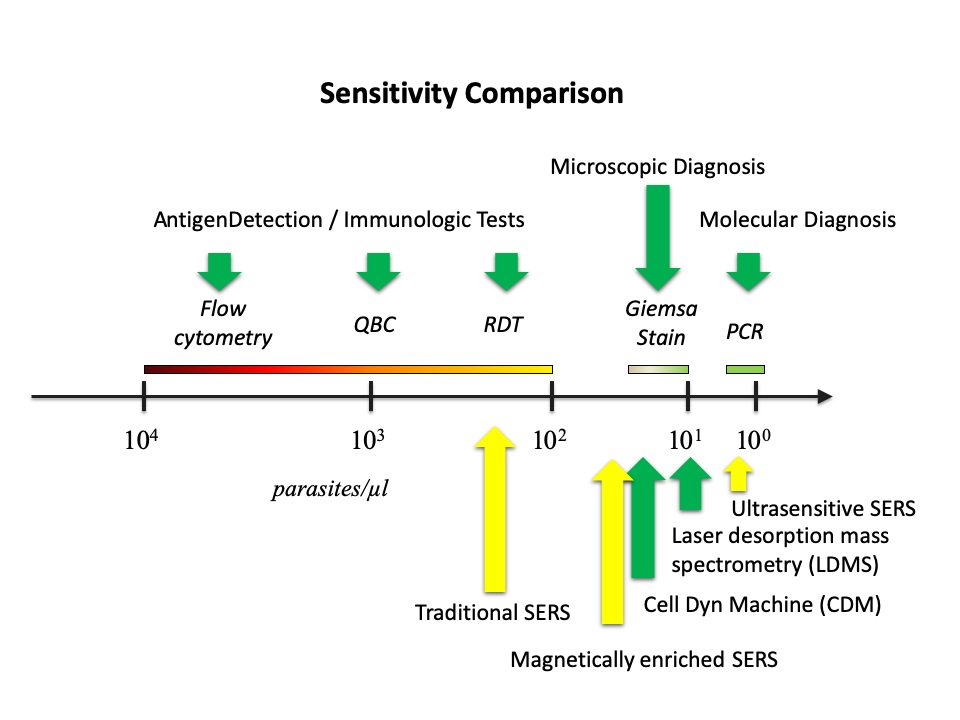
Fig.4 Comparison of the current techniques for malaria diagnosis in terms of sensitivity.
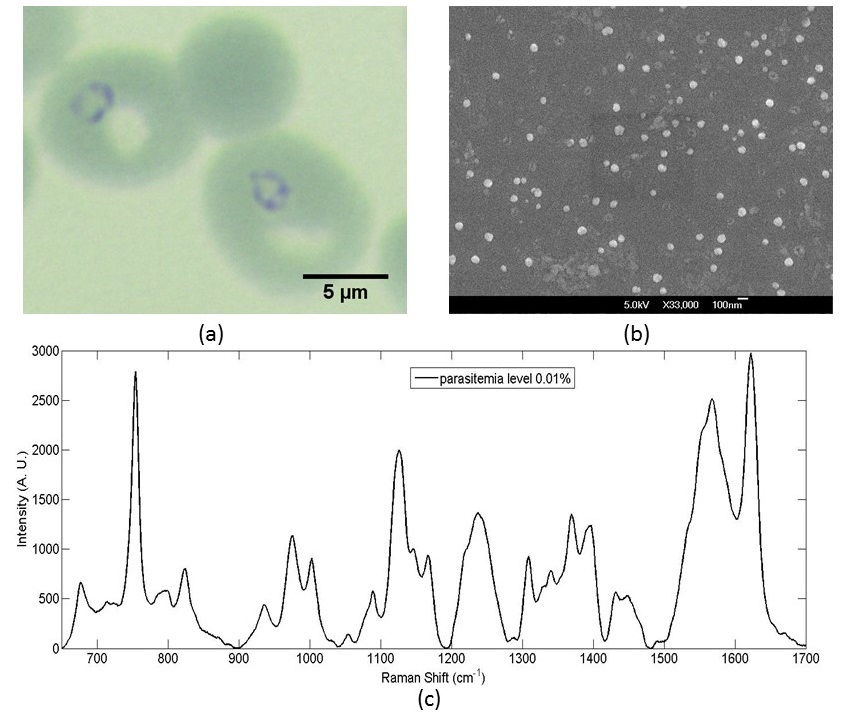
Fig. 5 (a) Gimesa stained images of ring-stage parasites; (b) SERS-active metal nanoparticles for SERS measurements in malaria diagnosis; (c) SERS spectrum of hemozoin after sample preparation.
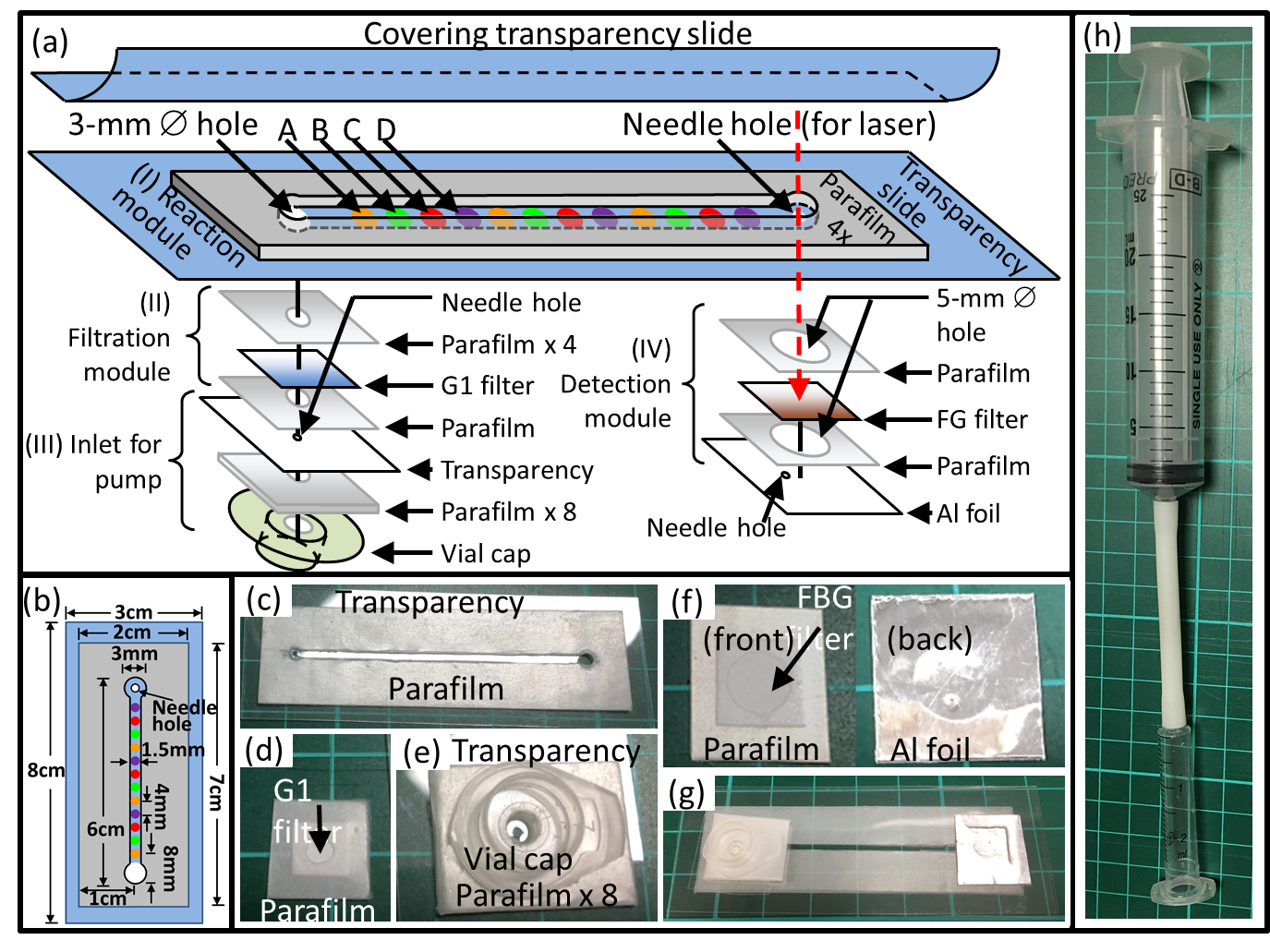
Fig. 6 Schematic drawings and various subassemblies photos of the SERS fluidic chip. (a) Partially exploded schematic and the constituent subassembly components of the chip: (I) reaction module, with 3 sets of dried chemical spots A, B, C, and D; (II) filtration module; (III) inlet for pump; and (IV) detection module. (b) Detailed dimension of (I) reaction module. (c)-(f) Subassembly photos that corresponding to module (I-IV), respectively. (g) Photo of the entire assembled SERS lab-on-chip. (h) Photo of our manual syringe pump. A: Silver Nitrate; B: Sodium Hydroxide C: Hydroxylamine Hydrochloride; D: Sodium Chloride; Al: Aluminum; FG filter: Fiber glass filter paper; G1 filter: Grade 1 filter paper. All holes in this chip are 3 mm in diameter,unless stated as needle hole (0.81mm ),or hole with diameter of 5mm. The needle hole on the Al foil in the detection module does not align to the laser path, while the other needle hole (for laser) on the transparency in the reaction module is intended for passing the laser beam for SERS measurements.
The above method requires blood to be drawn out of patients, which can cause significant inconvenience in sample preparation. Our group developed a stainless-steel microneedle based probe for intradermal measurements (Journal of Biophotonics 2014, selected as the Editor's Choice) as shown in Fig. 7&8, which could eliminate the need of drawing blood out of human body. Our group further developed a deformable agarose microneedle to reduce the risk of sharp injury and cross contamination due to needle reuses (Journal of Biomedical Optics 2015). For applications requiring microneedles with greater strength, we recently demonstrated that a plastic microneedle array can be modified to a surface enhanced Raman spectroscopy based biosensor for minimally invasive in vivo glucose measurements from living mice (ACS Sensors 2020) as shown in Fig. 9.
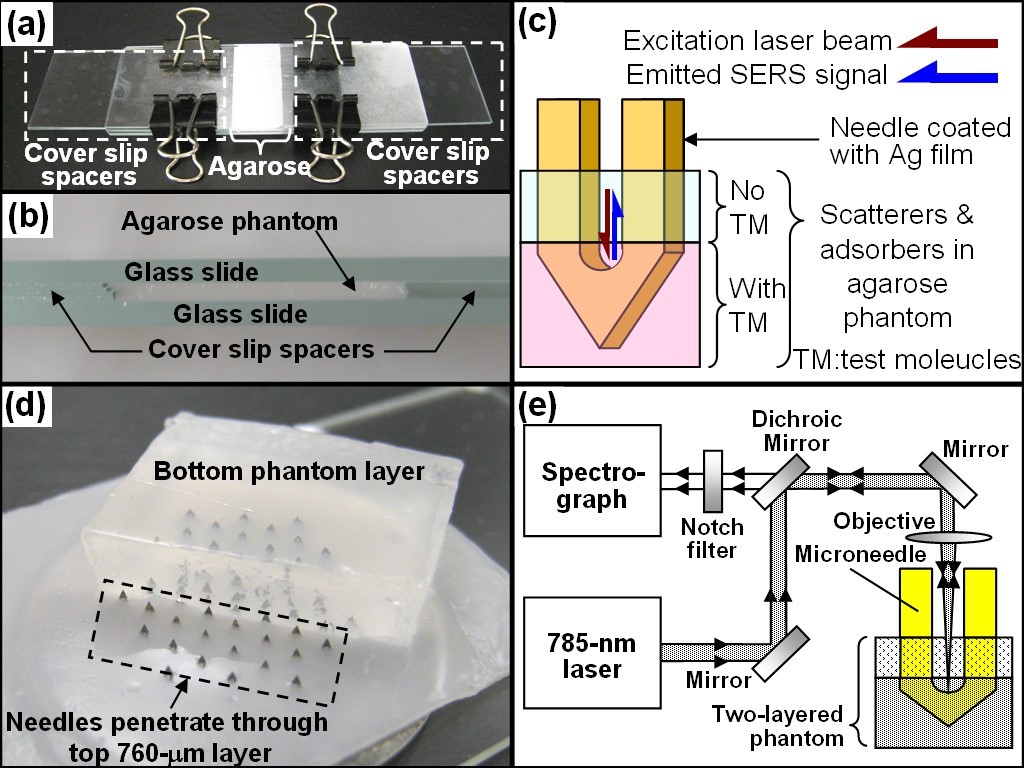
Fig. 7 (a) Image and (b) side view of the setup for the fabrication of 760-μm thick top phantom layers mimicking the skin. (c) Schematic of the Ag film coated microneedle probed into a two-layered phantom for intradermal measurements. (d) Microneedles penetrating through the top layer into the second layer. Note the phantom was placed upside down to facilitate the visualization of penetration. (e) Schematic of the Raman system for SERS measurements.
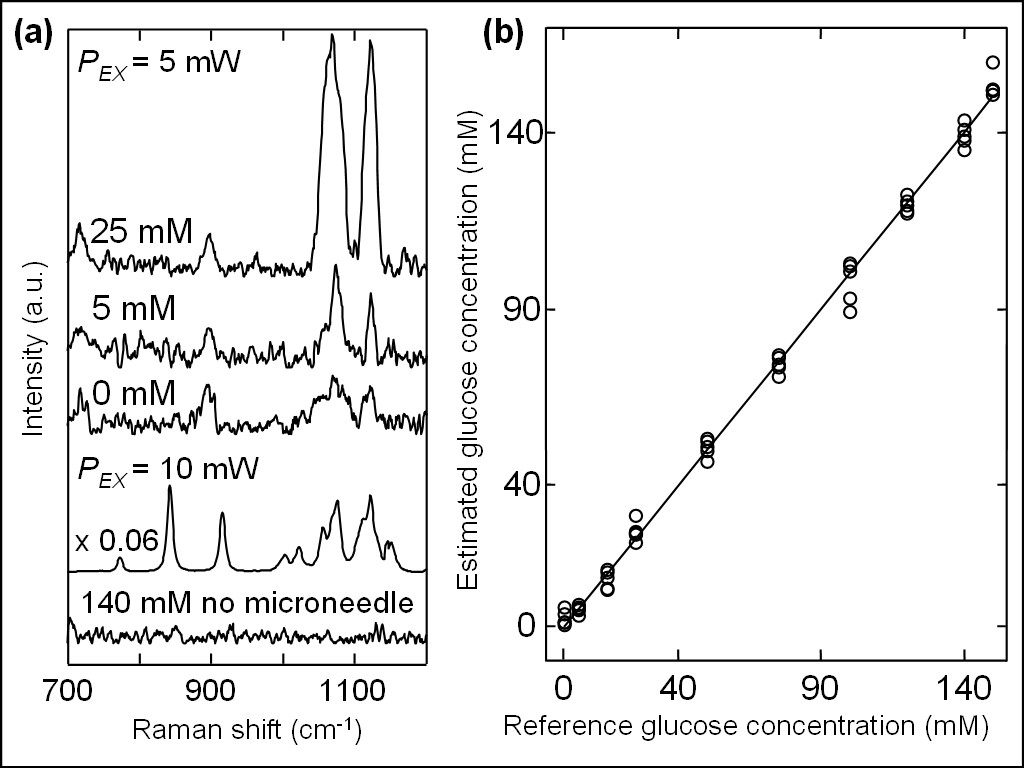
Fig. 8 (a) SERS spectra for the glucose concentrations of 0, 5, and 25 mM positioned inside phantom at 760 μm below the surface measured by using the Ag-coated microneedle-based probe at an excitation power of 5 mW, and ordinary Raman of crystalline glucose and glucose phantom at concentration of 140 mM without microneedle at an excitation power of 10 mW. (b) Correlation between estimated glucose concentrations and reference glucose concentrations, in which the former values were estimated by PLS-LOO method from SERS spectra measured in phantom using the Ag-coated microneedle-based probe.
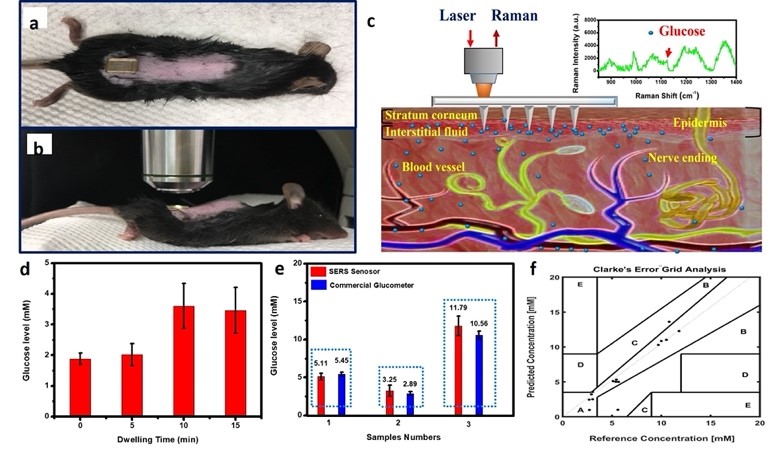
Fig. 9(a) Photograph of the F-PMMA MN array pressed onto the skin on the back of a mouse. (b) Mouse under anesthesia on the stage of the Raman microspectroscopy system for measurements. (c) Schematic illustration of the glucose measurement using the F-PMMA MN array for in vivo transdermal detection based on surface enhanced Raman spectroscopy. (d) Glucose level measured using SERS biosensor for a range of dwelling time from 0 to 15 min. (e) Glucose levels measured using our SERS glucose biosens or (red) in comparison with those obtained from a commercial glucometer (blue). We selected three mice with different blood glucose concentrations for testing in which the excitation power was 16.5 mW out of the needle tip at 785 nm and the exposure time was 10000 ms. Each mouse was tested sequentially for 5 times. (f) A Clarke error grid analysis of the in vivo glucose measurements using our SERS glucose biosensor in a mouse model of STZ- induced type I diabetes.
Relevant publications:
11.Clement Yuen, Xiaohong Gao, Prem Prakash, Yong Jia Ming, Chalapathy Raja Shobana, Perera Adhikarige Taniya Kaushalya, Luo Yue Mei, Bai Yan Ru, Charles Yang Chun, Peter Preiser, and Quan Liu*, "Towards malaria field diagnosis based on surface-enhanced Raman scattering with on-chip sample preparation and near-analyte nanoparticle synthesis," Sensors & Actuators: B. Chemical, 343, 130162 (2021)
10.Jian Ju, Chao-Mao Hsieh, Yao Tian, Jian Kang, Ruining Chia, Hao Chang, Yanru Bai, Chenjie Xu, Xiaomeng Wang, and Quan Liu*, "Surface Enhanced Raman Spectroscopy Based Biosensor with a Microneedle Array for Minimally Invasive In Vivo Glucose Measurements," ACS Sensors, 5(6), 1777-1785 (2020).
9.Jian Ju, Sagar Regmi, Afu Fu, Sierin Lim and Quan Liu*, "Graphene quantum dot based charge-reversal nanomaterial for nucleus-targeted drug delivery and efficiency controllable photodynamic therapy," Journal of Biophotonics, 12(6), e201800367, https://doi.org/10.1002/jbio.201800367 (2019) .
8.Jian Ju, Wei Liu, Clint Michael Perlaki, Keren Chen, Chunhua Feng and Quan Liu*, "Nitrogen doped Graphene Quantum Dots Effectively Preserve Surface Enhanced Raman Performance of Silver Nanoparticles," Under review (2017).
7. Keren Chen, Yi Hong Ong, Clement Yuen, Quan Liu*, "Surface-Enhanced Raman Spectroscopy for Intradermal Measurements", in Imaging in Dermatology, edited by Michael R Hamblin and Pinar Avci, Elsevier (2016) (ISBN: 978-0-12-802838-4), 141-154.
6. Keren Chen, Clint Perlaki, Aoli Xiong, Peter Preiser, and Quan Liu*, "Review of Surface Enhanced Raman Spectroscopy for Malaria Diagnosis and a New Approach for Detection of Single Parasites in the Ring Stage," IEEE Journal of Selected Topics in Quantum Electronics, 22(4), 6900509 (2016)(Invited Paper).
5. Keren Chen, Clement Yuen, Aniweh Yaw, Peter Preiser and Quan Liu*, "Towards ultra-sensitive malaria diagnosis using surface enhanced Raman spectroscopy," Scientific Reports, 6, 20177. (2016).
4. C. Yuen and Quan Liu*, "Hollow agarose microneedle with silver coating for intradermal surface enhanced Raman measurements: A skin-mimicking phantom study," Journal of Biomedical Optics, 20(6), 061102 (2015).
3. C. Yuen and Quan Liu*, "Towards in vivo intradermal surface enhanced Raman scattering (SERS) measurements: silver coated microneedle based SERS probe," Journal of Biophotonics, 7(9), 683-689 (2014) (Editor's Choice).
2. C. Yuen and Quan Liu*, "Optimization of Fe3O4@Ag Nanoshells for malaria diagnosis," Analyst 138(21), 6494-6500 (2013).
1. C. Yuen and Quan Liu*, "Magnetic Field Enriched Surface Enhanced Resonance Raman Spectroscopy for Early Malaria Diagnosis," Journal of Biomedical Optics 17(1), 017005 (2012).
Funding Sources: